IVC Filter Lawsuits
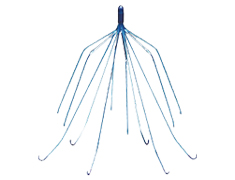
The IVC filter attorneys of McSweeney / Langevin are providing free legal consultations to individuals and families injured or harmed as a result of the implantation of an Inferior Vena Cava (IVC) Filter, a device that is commonly used to prevent pulmonary embolism.
If you or a loved one has been injured by an IVC filter, call 1-877-542-4646 or use our private online form to receive a free, confidential legal consultation.
Reports have shown defective IVC filters may break apart and travel through the body.
The IVC filter was introduced in 2005 to prevent pulmonary embolism in patients who cannot be treated through anticoagulation therapy. Unfortunately, over the last decade, the FDA has received nearly 1,000 reports of adverse patient outcomes caused by defective IVC filters. These filters have been found to fragment and cause embolisms in the body.
Due to recent research into these devices and reports made by physicians and consumers, the FDA has told physicians who recommend the use of IVC Filters to consider the risk that they pose to individual patients instead of choosing the IVC for all patients that are ineligible to receive anticoagulation therapy. Doctors have also been advised to remove the filters once symptoms have diminished.
A study performed at York Hospital in Pennsylvania looked at the safety of implanted IVC filters. The study found that 16% of devices implanted had fractured. Of these fractured pieces, nearly three-quarters had traveled to the heart where they were capable of causing serious side effects including rapid heartbeat, fluid buildup, and sudden death.
Types of IVC Filters
Common IVC Filter manufacturers include: Bard Peripheral Vascular, B Braun Medical, Boston Scientific, Cook Medical, Cordis, and Rafael Medical Technologies.
IVC Filter Lawsuits have already been filed
Numerous IVC Filter lawsuits against Bard Peripheral Vascular, Inc. and Cook Medical have already been filed. These IVC Filter lawsuits are so numerous they have been consolidated before a single judge for pretrial discovery. This type of lawsuit, known as multidistrict litigation, saves clients time and money by streamlining the legal process. It is also better than a class action lawsuit because every plaintiff has their own case that reflects the particular way that they were injured.
Recent IVC Filter Updates:
Filter Manufacturer Continued Selling Product it Knew to be Defective
A recent NBC News investigation found that C.R. Bard, a major IVC filter manufacturer, continued selling filters even after it knew they were defective. Confidential company records obtained by NBC News show New Jersey-based medical device giant C.R. Bard was concerned about reports of failures for its G2 series filters, designed to replace the company’s Recovery filter, within four months of being cleared to sell the G2 by the Food and Drug Administration.
Instead of recalling the G2 filter, and the virtually identical G2 Express, the medical device manufacturer decided to keep them on the market for five years, until 2010, selling more than 160,000 of them.
At least 12 deaths and hundreds of problems are now linked to the G2 series filters, according to Bard and FDA records.
The spider-shaped Bard filters (Bard Recovery Filter and Bard G2 Filter), implanted in the largest vein in the body, called the inferior vena cava, were designed to stop blood clots from moving to the heart and lungs, where they could be fatal.
Blood clot filters are implanted in an estimated 250,000 people in the U.S. each year, most without incident. In the last decade, millions of filters have been implanted in Americans. Bard is one of 11 manufacturers of the devices.
Bard hoped to gain a new foothold in the lucrative filter market when it introduced the Recovery filter, but after Bard received FDA clearance to market the IVC Filter in 2002, reports of deaths and injuries associated with the filter moving and breaking steadily increased.
A confidential study commissioned by Bard showed the Recovery filter had higher rates of relative risk for death, filter fracture and movement than all of its competitors. An outside doctor hired to conduct the study wrote “further investigation is urgently warranted.”
However, Bard decided not to recall the Recovery filter from the market. In 2005, after the device had been sold for three years, the company replaced it with the similar G2 series of filters. Internal Bard records and hundreds of reports to the FDA show the G2 series did not solve the filter’s problems.
A confidential memo written in December 2005 by a Bard vice president soon after the G2 was cleared by the FDA shows his concern regarding “problems with…migration,” “tilting” and “perforation.” He also noted Bard had another filter on the market with virtually no complaints. “Why shouldn’t doctors be using that one rather than the G2?”
Another document written on a later date includes data through 2010 showed the G2 series filters had more fractures, migrations and reported problems than any of its competitors.
In 2010 and 2014, the FDA recommended in safety alerts that doctors should consider removing the IVC filters from patients as soon as protection from blood clots is no longer needed.
The Society of Interventional Radiologists, Society for Vascular Surgery, and blood clot filter manufacturers, including Bard, have just begun a large clinical trial called PRESERVE to examine how safe and effective filters now on the market are. The study, which the FDA helped organize, is expected to enroll 2,100 patients over the course of five years, the most ambitious filter study ever in the U.S.
We Can Help
You may be entitled to compensation for medical bills, lost wages, pain and suffering and other damages if you or a loved one has suffered any adverse side effects due to a defective IVC Filter. Feel free to contact an IVC Filter attorney at 1-877-542-4646 or by using the form below. Your information will remain confidential and an IVC lawyer will provide you with a free legal consultation.
McSweeney / Langevin is willing to provide a consultation to individuals and families that have Bard, Cook, or other IVC filters. Our attorneys are even willing to provide a legal consultation if the IVC filter has not yet malfunctioned, or is still in place.

1-877-542-4646
FREE Case Review
Free Confidential Case Evaluation
To contact us for a free review of your potential case, please fill out the form below or call us toll free 24 hrs/day by dialing 1-877-542-4646
An asterisk (*) indicates a required field.














